Protease Activators
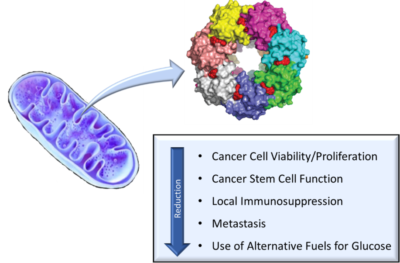

Madera has developed an extensive chemistry program aimed at targeting the caseinolytic protease P (ClpP). ClpP is part of the ClpXP complex, which is located in the mitochondrial matrix and is responsible for protein quality control. Professor Lee Graves of UNC and Madera identified the first highly potent small molecule activators of ClpP, known as the “TR Compounds.” Additional studies demonstrated how disruption of mitochondrial function through ClpP activation resulted in cancer growth inhibition in both cell and animal models. Madera’s collaborators at the NIH/NCI and UNC have also characterized the effects of the TR Compounds on cancer stem cell function. Most recently, Professor Houry of the University of Toronto and Madera have reported on the characterization of the interactions between a series of TR Compounds and ClpP.
Madera’s small molecules are designed to be exquisitely specific to ClpP, have high oral bioavailability, excellent cell permeability, and meet rigorous large pharma-like safety standards. Madera, UNC, and others have described one of Madera’s lead agents, TR-107, in recent publications. A portfolio of families of patents and patent applications protects Madera’s unique chemical library and discoveries.
Protein structure images provided by Professor Houry

